Welcome!
Welcome to the new CancerGRACE.org! Explore our fresh look and improved features—take a quick tour to see what’s new.

From the GRACE Archives - Originally Published August 26, 2009 | By Dr Pennell
OK, I hope you all aren’t bored to tears by this topic by now, given two related posts in April and again earlier this month describing the “broken” clinical trials system and the challenges to performing clinical research in the current environment. However, I thought this would be a good follow up post to let all of you know that the laughably obstructionist policies in place that prevent timely processing of clinical trials are being recognized, and that something is being done to fix it.
Clinical trials designed to test new cancer therapies are the only way that potentially effective drugs can make if from research labs into cancer patients in dire need of help. In April I talked about a couple of recent papers (see link above) that documented the complicated process of opening a clinical trial, involving hundreds of distinct processes in the bureaucratic morass of review, and taking multiple years from submission to trial opening.
In an early release publication in the Journal of Clinical Oncology, a group led by Dr. Razelle Kurzrock at the M.D. Anderson Cancer Center in Houston describes a new streamlined process for getting a phase I trial approved and the first cancer patient enrolled in what must be a record time (aptly called “Project Zero Delay”). This paper begins with a very interesting review of the myriad problems with the current system. For example, the authors note an average of 4-5 years for a company to take a new drug from the lab through enough initial safety studies in animals to get to clinical trials. After that, there is another 7.5 years before a promising drug makes it to FDA approval! All of this while over half a million people per year are dying of cancer in the USA, and of course millions elsewhere. The group then describes many of the same details I mentioned in my initial post about the delays in getting a specific trial opened.
Project Zero Delay focuses just on this aspect of the problem, namely getting a single new trial open. MD Anderson took it upon itself to also look at the exorbitant delay in opening trials, and designed a plan to fix it. The first step MD Anderson took was to negotiate a master agreement with a particular pharmaceutical company that would cover all trials over a 3-5 period, designed to overcome the months of budget and contract negotiations that normally take place for a new trial.
Most of the remaining problems with this process can be related to sequential levels of bureaucracy. In other words, drug companies, academic institutions, and the federal government each have an enormous amount of steps necessary to produce what is known as an Investigational New Drug (IND) application and then to open the trial, and these operations are usually done sequentially (one after the other) rather than simultaneously to be efficient. Project Zero Delay was designed to have all regulatory processes happen in parallel rather than sequentially, so that when the final OK came from the FDA the trial could open immediately.
The protocol described in the paper was designed cooperatively with the company and the MD Anderson principle investigator (PI). As the PI of a number of trials myself, I can tell you that the way it normally works is I write a protocol and then send it back to the company for review. This can then start a back and forth review and re-review process that can literally take years. In this paper, the authors were able to streamline this by having the company and PI cooperate at the inception of the concept so that the protocol could be completed fast.
Another sticking point is IRB (Institutional Review Board) review. Normally this necessary safety step, done to insure the safety of the study patient population, does not happen until the FDA has reviewed and approved a new IND for the study, adding months of time. In Project Zero Delay, the MD Anderson IRB agreed to review and approve the protocol PRIOR to FDA approval, so that when the IND arrived the trial was ready to open. I won’t go into every step, but all of the other regulatory steps also happened in parallel so that they all finished at the same time, without eliminating any steps! Look at the figures below. The first shows the normal process and timeline, while the second shows how all of the processes can be done simultaneously.
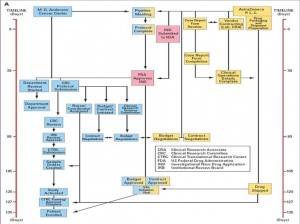
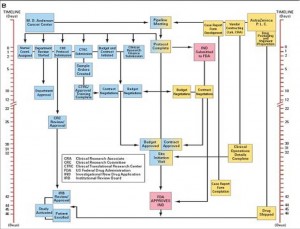
The final result is that the trial took only 46 days from protocol submission to enrolling the first patient on trial, and only 2 days from IND approval from the FDA! A month and a half. Not years, not six months, but six weeks! This is so amazing to me that I am still in a bit of shock and am still having trouble envisioning this applying broadly to every trial everywhere. I doubt every trial at MD Anderson moves this smoothly, and the paper doesn’t mention what level of personnel and resources are needed to make this work so quickly. But it does illustrate that with recognition of the problem, and with concerted effort to identify the issues and correct them, that clinical trials can be opened in a very reasonable amount of time. I am taking this paper to my boss and asking him if possibly we could do this too!
2 Comments
Please feel free to offer comments and raise questions in our
discussion forums.
An antibody–drug conjugate (ADC) works a bit like a Trojan horse. It has three main components:
Bispecifics, or bispecific antibodies, are advanced immunotherapy drugs engineered to have two binding sites, allowing them to latch onto two different targets simultaneously, like a cancer cell and a T-cell, effectively...
The prefix “oligo–” means few. Oligometastatic (at diagnosis) Oligoprogression (during treatment)
There will be a discussion, “Studies in Oligometastatic NSCLC: Current Data and Definitions,” which will focus on what we...
Radiation therapy is primarily a localized treatment, meaning it precisely targets a specific tumor or area of the body, unlike systemic treatments (like chemotherapy) that affect the whole body.
The...
Biomarkers are genetic mutations (like EGFR, ALK, KRAS, BRAF) or protein levels (like PD-L1) in tumor cells that help guide personalized treatment, especially NSCLC, directing patients to targeted therapies or immunotherapies...
Hi Stan! So good to hear from you. I'm sorry for the late response. I too have been out of town with family and missed your post, probably because I was...
It is so good to hear from you! And I am so happy to hear that your holidays have been good and that you are doing well. It sounds like your...

Welcome to the new CancerGRACE.org! Explore our fresh look and improved features—take a quick tour to see what’s new.
Catharine says:
August 26, 2009 at 4:52 pm
Dr. Pennell -
That is really impressive work by Dr. Kurzrock and her group at M.D. Anderson Cancer Center. I don’t want to be cliche, but “Where there’s a will, there’s a way.” Thank you for sharing this hopeful information. Best of luck on your efforts as well.
- Catharine
mo wanchuk says:
August 26, 2009 at 7:33 pm
Thanks for bringing this situation to light. We need development as quickly as science will go, not as quickly as bureacrats will go.