Welcome!
Welcome to the new CancerGRACE.org! Explore our fresh look and improved features—take a quick tour to see what’s new.
Earlier this week, I provided a brief review of several highlighted posters at ASCO, along with some commentary to place them into context. These posters represent only preliminary volleys of helpful information, describing findings from retrospective evaluations of outcomes from single institutions. Nevertheless, they provide a meaningful starting point for what should become a richer conversation as we come to understand how best to use targeted therapies for very targeted populations.
The main points I covered in my discussion were:
1) Is local treatment (surgery, radiation) feasible and potentially valuable in patients who develop acquired resistance to either EGFR tyrosine kinase inhibitors (TKIs) or an ALK inhibitor?
2) Might progression in the brain represent a "special case"?
3) Once a new systemic therapy is felt to be needed, should the targeted therapy be continued along with the new one (typically chemotherapy) or not?
4) Is there a potential benefit in retreating after time off of a targeted therapy, following its discontinuation for acquired resistance?
5) Is there any clinical relevance to the particular mechanism of acquired resistance, specifically the presence or absence of a T790M mutation (the most common cause for acquired resistance, a secondary resistance mutation seen in about 50-60% of cases once progression develops on an EGFR TKI)?
When considering these questions, I think it's helpful to recognize that there are a few different patterns of progression that are all called "acquired resistance" but are arguably very different situations.
-the patient with just a single area of progression or new lesion, but the vast majority of the person's cancer remains extremely well controlled.
-the patient with several areas of progression, but the pace of the disease is indolent, and there is still less disease than there was prior to starting targeted therapy.
-the patient who has multiple areas of progression growing rapidly
In an attempt to tackle the first two questions now, there were a few data sets that can help us a bit with the question of whether some patients might benefit from doing nothing initially but adding a local treatment and continuing the ongoing targeted therapy.
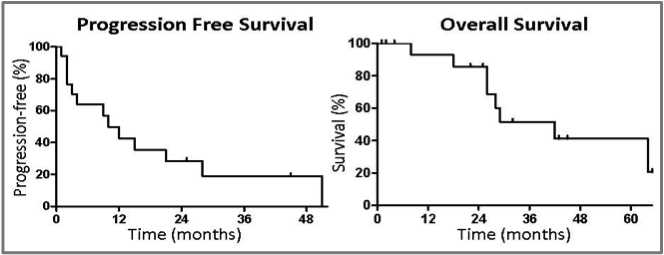
The first is by Drs. Yu and colleagues from Memorial Sloan-Kettering, who have been focusing research efforts on patients with an EGFR mutation since they were first described in 2004. They reviewed results from 184 patients who had an activating EGFR mutation and then acquired resistance, among whom 18 were treated with local therapy such as radiation or surgery, presumably because they demonstrated only a very limited extent of progression. They actually excluded patients with brain metastases, since they felt that this was a group for which local therapy was routinely recommended anyway (we don't know how many patients of the 184 would have qualified for this review by showing "brain only" or "brain first" progression). What they found was that in this small cohort, just 10% of the population of patients with an EGFR mutation and acquired resistance, was that they went a median of 10 months before any further progression, a median of 22 months before they were felt by their oncologist to need any further systemic treatment, and had a median overall survival from progression of nearly three years, at 34 months.
(see above image)
As you can see, there are some people who are experiencing very prolonged good results over a course of years here, even with no further intervention beyond a local treatment for a limited area of progression, along with ongoing EGFR inhibitor therapy.
In fact, the poster by Geoff Oxnard and colleagues provides more detail about some of these patients. This poster focused on a subset of pateints at Memorial Sloan-Kettering who had an EGFR mutation, developed acquired resistance, and were found on rebiopsy to have a secondary T790M mutation (many of these patients are the same ones included in the group from the Yu poster, described above). Several of these patients continued to do very well with ongoing EGFR TKI therapy and no other intervention except for radiation or surgery to an area of limited progression, as illustrated by the figure below, in which the length of the solid bars extending horizontally from the far left represent the duration of the first interval on an EGFR TKI before the first evidence of progression, the hatched bars extending to the right represent the duration on EGFR TKI therapy after initial progression but before it was felt that another systemic therapy was needed, and the circles with S or R represent the time point at which surgery or radiation, respectively, was administered.
 The results for a different cohort of patients from the University of Colorado was presented by Weickardt and colleagues. They actually pooled results from patients with either an activating EGFR mutation or ALK rearrangement; 51 of 65 patients with an EGFR mutation or ALK rearrangement had demonstrated acquired resistance, of whom 25 (49%) were considered to have disease limited enough to pursue local therapy, which the oncologists in Colorado defined as having progression in the brain (though excluding patients with leptomeningeal carcinomatosis) or progression in 4 or fewer sites outside of the brain. With these criteria, they found that their patients went a median of about 4 months on ongoing targeted therapy before progressing further if the initial progression was outside of the brain, and patients with initial progression in the brain went a median of 7 months after local therapy and ongoing targeted therapy before progressing further. In the curves below, the black line represents the time before patients showed initial progression, and the red line represents the time before subsequent progression after ongoing targeted therapy, combined with local therapy, was detected.
The results for a different cohort of patients from the University of Colorado was presented by Weickardt and colleagues. They actually pooled results from patients with either an activating EGFR mutation or ALK rearrangement; 51 of 65 patients with an EGFR mutation or ALK rearrangement had demonstrated acquired resistance, of whom 25 (49%) were considered to have disease limited enough to pursue local therapy, which the oncologists in Colorado defined as having progression in the brain (though excluding patients with leptomeningeal carcinomatosis) or progression in 4 or fewer sites outside of the brain. With these criteria, they found that their patients went a median of about 4 months on ongoing targeted therapy before progressing further if the initial progression was outside of the brain, and patients with initial progression in the brain went a median of 7 months after local therapy and ongoing targeted therapy before progressing further. In the curves below, the black line represents the time before patients showed initial progression, and the red line represents the time before subsequent progression after ongoing targeted therapy, combined with local therapy, was detected.
The authors suggested that the better results for ongoing treatment with targeted therapy after "brain first" or "brain only" progression is because this might represent a special case in which the progression isn't really because the cancer has become resistant, but rather that the drug levels of the targeted therapy at a standard daily schedule (or twice daily for XALKORI (crizotinib)) doesn't penetrate into the central nervous system (CNS) well enough to have a meaningful effect -- and there is some evidence that supports that idea that drug levels in the cerebrospinal fluid are very low -- too low to be expected to have any effect like you see in the rest of the body. This is also supported by some data out of Kobe, Japan that looked at patients with an activating EGFR mutation who underwent rebiopsy after progression in the setting of acquired resistance, finding that the rate of secondary resistance T790M mutations was just 10% for disease that was detected in the CNS, compared with a 60% rate of T790M mutations for progressing lesions outside of the CNS. The Japanese authors hypothesized that this is because brain lesions develop not because they're under "selective pressure" of active treatment against the EGFR mutation, but rather because the CNS is a "sanctuary site" in which the disease in the brain remains essentially unexposed to EGFR TKI therapy.
The implication is that the EGFR TKI therapy can still be very effective against the disease it's reaching, while local therapy to the brain -- generally radiation -- can be used to treat the sanctuary site. This is why it might make sense to consider progression in the brain as a "special case" that doesn't necessarily suggest a patient should discontinue their targeted therapy.
In my discussion at ASCO, I noted that the outcomes with this strategy of continued targeted therapy and local treatment for progression was far, far better in the Memorial Sloan-Kettering experience, and I think this is because it was applied in a much more selected group of patients -- just 10% of those with acquired resistance, compared with almost exactly half of the patients in the Colorado experience. This suggests to me that if there might be a benefit to local treatment, it is only for a real minority of cases, and applying it far more broadly will really water down our ability to determine whether there really might be a population very well served by this strategy. An alternative explanation is that these patients with very limited progression also just happen to be the patients destined to do the best anyway, so it may be that these patients who are the best candidates for local therapy are the very ones who would do just as well or better if we could manage to ignore the imaging showing very limited progression.
We'll turn next to the question of what to do when there is more multifocal progression and it's felt that it's time to bring in chemotherapy: should the targeted therapy be continued with the chemo concurrently, or is it better to stop it, give a break from the targeted therapy, and perhaps revisit trying the targeted therapy again later. Look for that discussion very soon.
Please feel free to offer comments and raise questions in our
discussion forums.
Dr. Singhi's reprise on appropriate treatment, "Right patient, right time, right team".
While Dr. Ryckman described radiation oncology as "the perfect blend of nerd skills and empathy".
I hope any...
My understanding of ADCs is very basic. I plan to study Dr. Rous’ discussion to broaden that understanding.

An antibody–drug conjugate (ADC) works a bit like a Trojan horse. It has three main components:
Bispecifics, or bispecific antibodies, are advanced immunotherapy drugs engineered to have two binding sites, allowing them to latch onto two different targets simultaneously, like a cancer cell and a T-cell, effectively...
The prefix “oligo–” means few. Oligometastatic (at diagnosis) Oligoprogression (during treatment)
There will be a discussion, “Studies in Oligometastatic NSCLC: Current Data and Definitions,” which will focus on what we...
Radiation therapy is primarily a localized treatment, meaning it precisely targets a specific tumor or area of the body, unlike systemic treatments (like chemotherapy) that affect the whole body.
The...

Welcome to the new CancerGRACE.org! Explore our fresh look and improved features—take a quick tour to see what’s new.
A Brief Tornado. I love the analogy Dr. Antonoff gave us to describe her presentation. I felt it earlier too and am looking forward to going back for deeper dive.